Abstract

Background: High dose methotrexate (HDMTX), defined as doses > 500-1000 mg/m2, is used to treat a variety of adult and pediatric malignancies. HDMTX can cause significant nephrotoxicity. Volume depletion and acidic urine are major risk factors for acute kidney injury. Precipitation of MTX crystals is most likely to occur in acidic urine when pH drops below 5.5. Increasing urine pH from 6.0 to 7.0 has been demonstrated to increase the solubility of MTX and its metabolites by five to eight-fold. Continuous intravenous (IV) sodium bicarbonate has been a simple long term solution for urinary alkalization allowing for rapid titration to accommodate fluctuations in urinary pH.1-2
A recent breakdown in the supply chain of IV sodium bicarbonate has become a healthcare crisis compromising patient care. Sodium bicarbonate (8.4% and 7.5%) 50mL syringes are estimated to remain on back order until at least August 2017. Alternative sources, such as sodium acetate, are in limited supply as well.3-4 In a small case series, acetazolamide 500 mg IV q6H was reported to achieve adequate urinary alkalization, however efficacy of such a regimen has not been examined in a large population.5 Our lack of IV sodium bicarbonate created an urgent need to establish a practical alternative for urinary alkalization in patients receiving HDMTX.
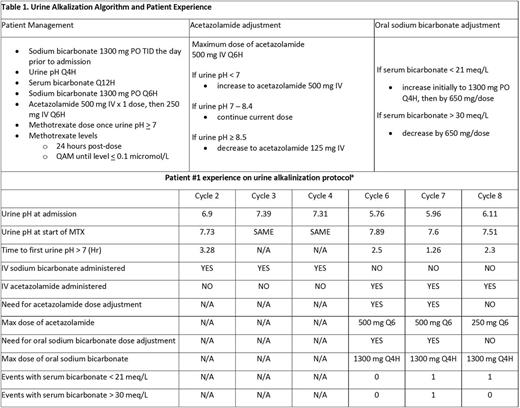
Methods: We designed a pilot algorithm for urinary alkalization in patients undergoing HDMTX (table 1). The algorithm relies on administration of IV acetazolamide to induce urinary alkalosis in combination with oral sodium bicarbonate to mitigate the metabolic acidosis induced by acetazolamide.5 MTX is not administered until urinary alkalization (urine pH > 7) is achieved, and the algorithm is continued until serum MTX levels are confirmed to be < 0.1 micromol/L. Additional supportive measures including hydration and leucovorin rescue are routinely provided per our institutional practice. Parameters for additional supportive measures are not defined by the algorithm and dose adjustment of leucovorin was left to the discretion of the treating physician in collaboration with the hematology/oncology clinical pharmacist.
Results: Our algorithm was utilized in a patient on three separate encounters, all of which involved administration of HDMTX (8 gm/m2 IV) for primary central nervous system lymphoma. Time to urine pH > 7 using acetazolamide was < 2.5 hours allowing prompt initiation of therapy on admission. Urine pH < 7 was seen once while on IV sodium bicarbonate compared with a total of five times on our pilot algorithm; however the urine pH never dropped below 6.5 on our algorithm (table 1). Average length of stay was similar between both groups (five days with IV sodium bicarbonate and 4.67 days with our algorithm). No cycles resulted in elevations of serum creatinine, liver function tests, or mucositis. Utilizing a maximum dose of IV acetazolamide and oral sodium bicarbonate required for these patients, wholesale acquisition drug cost comparison for 24 hours of therapy is similar for both strategies.
Conclusions: In summary, our pilot algorithm for urine alkalization demonstrated that using IV acetazolamide with oral sodium bicarbonate is a tolerable and effective alternative to the use of continuous sodium bicarbonate infusion when treating patients with HDMTX. The algorithm allowed safe treatment of patients with HDMTX at our institution during a period of no access to intravenous sodium bicarbonate. Our observations include prompt urine alkalinization, effective control of urine pH > 7, ease of administration and tolerability, and rapid reductions in plasma MTX concentrations. Limitation to the widespread use of acetazolamide includes metabolic acidosis though not seen in this limited experience. Notwithstanding this limitation, our algorithm demonstrates a viable alternative to sodium bicarbonate infusion and clearly merits further investigation. With the ongoing lack of access to IV sodium bicarbonate, we continue to utilize the algorithm for additional patients undergoing treatment with HDMTX.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal